Leaving Community
Are you sure you want to leave this community? Leaving the community will revoke any permissions you have been granted in this community.
Cathepsin_S - Overview
Challenge timeframe: Sep 04, 2018 to Dec 04, 2018
Updates
2019-02-21 - Preliminary Affinity Ranking results are now available on the "Data Download" page. You must be logged in first to access the files.
2018-12-07 - Answers to the challenges are now available: CatS_score_compounds_D3R_GC4_answers.csv and CatS_FEset_compounds_D3R_GC4_answers.csv
2018-11-27 - Submissions are now being accepted. Note: structure based scoring files that exceed 20M will likely not work for now. In the meantime, please upload your file to a public server like box.com, google drive and email us the download link. In it's place, please upload a smaller version to the D3R website that we can manually replace.
2018-11-26 - Template and example files are ready for download
2018-10-29 - Please note that the CatS ligands numbering in the free energy and scoring sets are offset by one.
A sharp-eyed participant noticed that the names of the ligands in the CatS free energy sets don't match those in the full scoring set. Please keep the naming as is; we will account for the discrepancy in the evaluation process.
- CatS_target_D3R_GC4.fasta: Protein sequence file of the Cathepsin S construct used.
- CatS_score_compounds_D3R_GC4.csv: CSV file of 459 compounds and their corresponding SMILES string.
- CatS_FESet_compounds_D3R_GC4.csv: CSV file of 39 compounds selected from CatS_score_compounds_D3R_GC4.csv for explicit-solvent relative or absolute free energy calculations.
Note: No attempt was made to set appropriate starting conformations or optimal protonation or tautomer states for the ligands, or to generate alternative tautomer states. It is up to you to choose and set these states for your calculations.
Introduction to Cathepsin S
The cathepsins constitute an 11-member family of proteases involved in protein degradation. Cathepsin S is highly expressed in antigen-presenting cells, where it degrades major histocompatibility complex class II (MHC II)-associated invariant chain. CatS is a candidate target for regulating immune hyper-responsiveness, as the inhibition of CatS may limit antigen presentation1-3.
This data set comprises a follow-on challenge to GC3, consisting of non-peptidic, non-covalent, small molecule inhibitors across a three order of magnitude range (nM to μM) of IC50s for CatS. Specifically, we provide 459 CatS inhibitors for affinity prediction, and 39 molecules for free energy prediction. This dataset was kindly donated by Janssen. Please note the affinity values from this set were measured against a C25S CatS mutant.
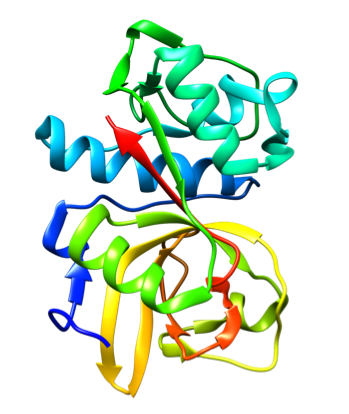
Representative crystal structures of CatS (PDB 2HHN).
Cathepsin S Binding assay conditions
For details of the binding assays, we were referred to publications from the Janssen group, which describe the following binding assays conditions4
References
- Ameriks MK, Bembenek SD, Burdett MT, et al (2010) Diazinones as P2 replacements for pyrazole-based cathepsin S inhibitors. Bioorg Med Chem Lett 20:4060-4064. doi: 10.1016/j.bmcl.2010.05.086
- Wiener DK, Lee-Dutra A, Bembenek S, et al (2010) Thioether acetamides as P3 binding elements for tetrahydropyrido-pyrazole cathepsin S inhibitors. Bioorg Med Chem Lett 20:2379-2382. doi: 10.1016/j.bmcl.2010.01.103
- Ameriks MK, Axe FU, Bembenek SD, et al (2009) Pyrazole-based cathepsin S inhibitors with arylalkynes as P1 binding elements. Bioorg Med Chem Lett 19:6131-6134. doi: 10.1016/j.bmcl.2009.09.014
- Thurmond RL, Sun S, Sehon CA, et al (2004) Identification of a Potent and Selective Noncovalent Cathepsin S Inhibitor. J Pharmacol Exp Ther 308:268-276. doi: 10.1124/jpet.103.056879
Cathepsin_S - Data Download
Challenge timeframe: Sep 04, 2018 to Dec 04, 2018
Cathepsin_S - Protocols
Challenge timeframe: Sep 04, 2018 to Dec 04, 2018
Please join the challenge and Login.
Cathepsin_S - Submissions
Challenge timeframe: Sep 04, 2018 to Dec 04, 2018
Please join the challenge and Login.
Cathepsin_S - Evaluation Results
Challenge timeframe: Sep 04, 2018 to Dec 04, 2018
Evaluation Results
Overviews
Last updated April 18, 2019Pose Predictions
Affinity Predictions
This section presents metrics of the ability of the predictions to correctly rank ligands by affinity. The rankings were evaluated in terms of the Kendall's τ and Spearman's ρ. Predicted binding energies for the free energy sets were additionally evaluated in terms of centered root-mean-square error (RMSEc, kcal/mol) [1] and Pearson's R. Uncertainties in these statistics (e.g., Kendall's τ Errors in the table) were obtained by recomputing them in 10,000 rounds of resampling with replacement, where, in each sample, the experimental IC50 or Kd data were randomly modified based on the experimental uncertainties. Experimental uncertainties are added to the free energy, ΔG, as a random offset δG drawn from a Gaussian distribution of mean zero and standard deviation RTln(Ierr). In this evaluation, the value of Ierr was set to 2.5.
User Submissions
BACEStage1a.zip (812M)
BACEStage1b.zip (383M)
BACEStage2.zip (786M)
CatS.zip (966M)
gc4protocols.zip (700k)
Further details of these procedures and results will be provided in an overview paper in the special issue of JCAMD.
All scripts used to evaluate submissions are publicly available on Github.
(partials) indicates submissions that do not include the full set of predictions